Short Stature Workup: Laboratory Studies, Imaging Studies, Other Tests
Short Stature Workup: Laboratory Studies, Imaging Studies, Other Tests
Laboratory studies used to assess the major causes of short stature in children include the following:
Other useful tests include the following:
Perform anteroposterior radiography of left hand and wrist to assess bone age (see image below).
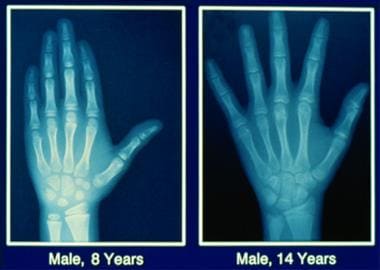
Bone age comparison between an 8-year-old boy (left) and a 14-year-old adolescent boy (right).
Chondrodysplasia of the distal radial epiphysis (Madelung deformity) suggests Lerí-Weill dyschondrosteosis.
Perform renal and cardiac ultrasonography in all patients with Ullrich-Turner syndrome. The most commonly associated anomalies include horseshoe kidney and bicuspid aortic valve.
Perform hearing tests in all patients with Ullrich-Turner syndrome. Use Bayley-Pinneau or Tanner-Goldstein-Whitehouse methods. These methods are often used to predict final adult height and become more accurate with advancing bone age.
Within 5 years of epiphyseal closure, the predicted height may fall within ±5 cm of the final adult height, with 95% confidence. The Bayley-Pinneau method can be used with a bone age as young as 6 years; however, the prediction is less accurate at the younger ages.
Several provocative tests have been developed for the evaluation of suspected GHD, including the following:
Perform all GH provocative testing under the supervision of a pediatric endocrinologist. Please refer to Hyposomatotropism for further details of these tests.
Treatment & Management
Sunil Sinha, MD Assistant Professor, Division of Pediatric Endocrinology and Metabolism, Department of Pediatrics, University of Tennessee Health Science Center
Sunil Sinha, MD is a member of the following medical societies: American Academy of Pediatrics, American Association of Clinical Endocrinologists, Endocrine Society, Pediatric Endocrine Society
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Lynne Lipton Levitsky, MD Chief, Pediatric Endocrine Unit, Massachusetts General Hospital; Associate Professor of Pediatrics, Harvard Medical School
Lynne Lipton Levitsky, MD is a member of the following medical societies: Alpha Omega Alpha, American Academy of Pediatrics, American Diabetes Association, American Pediatric Society, Endocrine Society, Pediatric Endocrine Society, Society for Pediatric Research
Disclosure: Received grant/research funds from Eli Lilly for pi; Received grant/research funds from NovoNordisk for pi; Received consulting fee from NovoNordisk for consulting; Partner received consulting fee from Onyx Heart Valve for consulting.
Chief Editor
Stephen Kemp, MD, PhD Professor, Department of Pediatrics, Section of Pediatric Endocrinology, University of Arkansas for Medical Sciences College of Medicine, Arkansas Children's Hospital
Stephen Kemp, MD, PhD is a member of the following medical societies: American Academy of Pediatrics, American Association of Clinical Endocrinologists, American Pediatric Society, Endocrine Society, Phi Beta Kappa, Southern Medical Association, Southern Society for Pediatric Research
Angelo P Giardino, MD, PhD, MPH Associate Professor, Baylor College of Medicine; Chief Medical Officer, Texas Children’s Health Plan; Chief Quality Officer, Medicine, Texas Children’s Hospital
Angelo P Giardino, MD, PhD, MPH is a member of the following medical societies: American Professional Society on the Abuse of Children, Harris County Medical Society, International Society for the Prevention of Child Abuse and Neglect, Ray E Helfer Society, Academic Pediatric Association, American Academy of Pediatrics
Disclosure: Received grant/research funds from Health Resources and Services Administration (HRSA) Integrated Community Systems for CSHCN Grant for other; Received advisory board from Baxter Healthcare Corporation for board membership.
Acknowledgements
Robert J Ferry Jr, MD Le Bonheur Chair of Excellence in Endocrinology, Professor and Chief, Division of Pediatric Endocrinology and Metabolism, Department of Pediatrics, University of Tennessee Health Science Center
Robert J Ferry Jr, MD is a member of the following medical societies: American Academy of Pediatrics, American Diabetes Association, American Medical Association, Endocrine Society, Pediatric Endocrine Society, Society for Pediatric Research, and Texas Pediatric Society
Disclosure: Eli Lilly & Co Grant/research funds Investigator; MacroGenics, Inc Grant/research funds Investigator; Ipsen, SA (formerly Tercica, Inc) Grant/research funds Investigator; NovoNordisk SA Grant/research funds Investigator; Diamyd Grant/research funds Investigator; Bristol-Myers-Squibb Grant/research funds Other; Amylin Other; Pfizer Grant/research funds Other; Takeda Grant/research funds Other
References
Proper use of a wall-mounted stadiometer.
Comparison of the growth patterns between idiopathic short stature and constitutional growth delay.
Bone age comparison between an 8-year-old boy (left) and a 14-year-old adolescent boy (right).
Growth chart for Turner syndrome. Note that the upper limit overlaps the range for girls of normal height.
A single, central, maxillary incisor reflects a defect in midline facial development.
Laboratory Studies
Laboratory studies used to assess the major causes of short stature in children include the following:
- Measurement of serum levels of insulinlike growth factor-I (IGF-I), formerly named somatomedin C, and IGF binding protein-3 (IGFBP-3)
- These are useful tests for growth hormone deficiency (GHD), except in pubertal patients and those with history of a brain tumor.
- Patients with certain CNS neoplasms may have normal serum growth factor levels despite having GHD, particularly during puberty.
- Consider provocative tests of pituitary function in any patient with normal thyroid function suspected to be GH deficient.
- Interpret a low serum IGF-I concentration cautiously because poor nutrition is associated with low serum IGF-I concentration.
- The serum IGFBP-3 concentration has greater specificity than serum IGF-I concentration in the diagnosis of GHD.
- Karyotype by G-banding
- The 45,X pattern defines patients with Ullrich-Turner syndrome.
- Because 10% of patients with Ullrich-Turner syndrome possess a mosaic karyotype (eg, 45,X; 46,XX), counting at least 30 cells reduces the possibility of failing to identify a patient with mosaic Turner syndrome (TS).
- Measuring serum levels of GH
- Beyond the first months of life, endogenous GH is secreted in a pulsatile fashion. These intermittent peaks are greatest after exercise, after meals (as blood glucose levels decrease), and during deep sleep. Therefore, measuring a single random serum GH value is of no use in the evaluation of the short child. Beyond the neonatal period, values obtained during the daytime are unlikely to be detectable.
- Although a random serum GH value of more than 10 mg/dL generally excludes GHD, a random low serum GH concentration does not confirm the diagnosis of GHD.
Other useful tests include the following:
- CBC count for hematologic disease
- Wintrobe sedimentation rate for inflammatory bowel disease
- Antiendomysial immunoglobulin A (IgA) and immunoglobulin G (IgG), transglutaminase IgG, and antigliadin IgG titers for sprue (gluten enteropathy) (Antiendomysial IgA titers are more sensitive, and IgG titers are more specific.)
- Serum total thyroxine (total T4) and thyrotropin (TSH) levels to test for hypothyroidism
- Determination of serum free T4 concentration is necessary in patients in whom TSH deficiency, TRH deficiency, or thyroxine-binding globulin (TBG) deficiency is suspected.
- Directly assay free T4 levels using equilibrium dialysis.
- Many reference laboratories report a value termed the free thyroxine index, which is calculated by multiplying the total T4 by an internal standard; however, if free T4 assessment is needed, measure it directly.
- Sweat chloride testing to exclude cystic fibrosis (CF): Consider this test in patients who are short and have a history of meconium ileus or pulmonary symptoms.
- Serum transferrin and prealbumin concentrations for undernutrition
Imaging Studies
Perform anteroposterior radiography of left hand and wrist to assess bone age (see image below).

Bone age comparison between an 8-year-old boy (left) and a 14-year-old adolescent boy (right).
Chondrodysplasia of the distal radial epiphysis (Madelung deformity) suggests Lerí-Weill dyschondrosteosis.
Perform renal and cardiac ultrasonography in all patients with Ullrich-Turner syndrome. The most commonly associated anomalies include horseshoe kidney and bicuspid aortic valve.
Other Tests
Perform hearing tests in all patients with Ullrich-Turner syndrome. Use Bayley-Pinneau or Tanner-Goldstein-Whitehouse methods. These methods are often used to predict final adult height and become more accurate with advancing bone age.
Within 5 years of epiphyseal closure, the predicted height may fall within ±5 cm of the final adult height, with 95% confidence. The Bayley-Pinneau method can be used with a bone age as young as 6 years; however, the prediction is less accurate at the younger ages.
Procedures
Several provocative tests have been developed for the evaluation of suspected GHD, including the following:
- Insulin-induced hypoglycemia is the most powerful stimulus for GH secretion; however, this test also carries the greatest potential for harm and is the only GH provocative test that has been associated with fatalities.
- Alternate GH secretagogues used successfully in combination as 2 serial tests include arginine, levodopa, propranolol with glucagon, exercise, clonidine, or epinephrine.
- Peak GH level is higher if the patient recently exposed to sex steroids but controversy among pediatric endocrinologist persists regarding the use of sex steroid priming prior to stimulation testing.
Perform all GH provocative testing under the supervision of a pediatric endocrinologist. Please refer to Hyposomatotropism for further details of these tests.
Treatment & Management
Sunil Sinha, MD Assistant Professor, Division of Pediatric Endocrinology and Metabolism, Department of Pediatrics, University of Tennessee Health Science Center
Sunil Sinha, MD is a member of the following medical societies: American Academy of Pediatrics, American Association of Clinical Endocrinologists, Endocrine Society, Pediatric Endocrine Society
Specialty Editor Board
Mary L Windle, PharmD Adjunct Associate Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Lynne Lipton Levitsky, MD Chief, Pediatric Endocrine Unit, Massachusetts General Hospital; Associate Professor of Pediatrics, Harvard Medical School
Lynne Lipton Levitsky, MD is a member of the following medical societies: Alpha Omega Alpha, American Academy of Pediatrics, American Diabetes Association, American Pediatric Society, Endocrine Society, Pediatric Endocrine Society, Society for Pediatric Research
Disclosure: Received grant/research funds from Eli Lilly for pi; Received grant/research funds from NovoNordisk for pi; Received consulting fee from NovoNordisk for consulting; Partner received consulting fee from Onyx Heart Valve for consulting.
Chief Editor
Stephen Kemp, MD, PhD Professor, Department of Pediatrics, Section of Pediatric Endocrinology, University of Arkansas for Medical Sciences College of Medicine, Arkansas Children's Hospital
Stephen Kemp, MD, PhD is a member of the following medical societies: American Academy of Pediatrics, American Association of Clinical Endocrinologists, American Pediatric Society, Endocrine Society, Phi Beta Kappa, Southern Medical Association, Southern Society for Pediatric Research
Angelo P Giardino, MD, PhD, MPH Associate Professor, Baylor College of Medicine; Chief Medical Officer, Texas Children’s Health Plan; Chief Quality Officer, Medicine, Texas Children’s Hospital
Angelo P Giardino, MD, PhD, MPH is a member of the following medical societies: American Professional Society on the Abuse of Children, Harris County Medical Society, International Society for the Prevention of Child Abuse and Neglect, Ray E Helfer Society, Academic Pediatric Association, American Academy of Pediatrics
Disclosure: Received grant/research funds from Health Resources and Services Administration (HRSA) Integrated Community Systems for CSHCN Grant for other; Received advisory board from Baxter Healthcare Corporation for board membership.
Acknowledgements
Robert J Ferry Jr, MD Le Bonheur Chair of Excellence in Endocrinology, Professor and Chief, Division of Pediatric Endocrinology and Metabolism, Department of Pediatrics, University of Tennessee Health Science Center
Robert J Ferry Jr, MD is a member of the following medical societies: American Academy of Pediatrics, American Diabetes Association, American Medical Association, Endocrine Society, Pediatric Endocrine Society, Society for Pediatric Research, and Texas Pediatric Society
Disclosure: Eli Lilly & Co Grant/research funds Investigator; MacroGenics, Inc Grant/research funds Investigator; Ipsen, SA (formerly Tercica, Inc) Grant/research funds Investigator; NovoNordisk SA Grant/research funds Investigator; Diamyd Grant/research funds Investigator; Bristol-Myers-Squibb Grant/research funds Other; Amylin Other; Pfizer Grant/research funds Other; Takeda Grant/research funds Other
References
- [Guideline] Cohen P, Rogol AD, Deal CL, et al. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. J Clin Endocrinol Metab. 2008 Nov. 93(11):4210-7. [Medline].
- Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J Pediatr. 1994 Jul. 125(1):29-35. [Medline].
- Dauber A, Rosenfeld RG, Hirschhorn JN. Genetic Evaluation of Short Stature. J Clin Endocrinol Metab. 2014 Jun 10. jc20141506. [Medline].
- Albertsson-Wikland K, Aronson AS, Gustafsson J, et al. Dose-dependent effect of growth hormone on final height in children with short stature without growth hormone deficiency. J Clin Endocrinol Metab. 2008 Nov. 93(11):4342-50. [Medline].
- Collett-Solberg PF, Misra M,. The role of recombinant human insulin-like growth factor-I in treating children with short stature. J Clin Endocrinol Metab. 2008 Jan. 93(1):10-8. [Medline].
- Cohen P, Germak J, Rogol AD, et al. Variable Degree of Growth Hormone (GH) and Insulin-Like Growth Factor (IGF) Sensitivity in Children with Idiopathic Short Stature Compared with GH-Deficient Patients: Evidence from an IGF-Based Dosing Study of Short Children. J Clin Endocrinol Metab. 2010 Mar 5. [Medline].
- Carel JC, Ecosse E, Landier F, Meguellati-Hakkas D, Kaguelidou F, Rey G, et al. Long-term mortality after recombinant growth hormonetreatment for isolated childhood short stature: report of the French SAGhE Study. Program of the 93rd Annual Meeting of The. 2011. Abstract LB-5.
- Sävendahl L, Maes M, Albertsson-Wikland K, Borgström B, Carel JC, Henrard S, et al. Long-term mortality and causes of death in isolated GHD, ISS, and SGA patients treated with recombinant growth hormone during childhood in Belgium, The Netherlands, and Sweden: preliminary report of 3 countries participating in the EU SAGhE study. J Clin Endocrinol Metab. Feb 2012. 97(2):E213-7. [Medline].
- US Food and Drug Administration. Recombinant Human Growth Hormone (somatropin): Ongoing Safety Review - Possible Increased Risk of Death. Available at http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm237969.htm?utm_campaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=growth hormone&utm_content=4. Accessed: June 2012.
- Hagman A, Wennerholm UB, Kallen K, et al. Women who gave birth to girls with Turner syndrome: maternal and neonatal characteristics. Hum Reprod. 2010 Apr 10. [Medline].
- Attie KM, Julius JR, Stoppani C, Rundle AC. National Cooperative Growth Study substudy VI: the clinical utility of growth-hormone-binding protein, insulin-like growth factor I, and insulin-like growth factor-binding protein 3 measurements. J Pediatr. 1997 Jul. 131(1 Pt 2):S56-60. [Medline].
- Badaru A, Wilson DM. Alternatives to growth hormone stimulation testing in children. Trends Endocrinol Metab. 2004 Aug. 15(6):252-8. [Medline].
- Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952 Apr. 40(4):423-41. [Medline].
- Belin V, Cusin V, Viot G, et al. SHOX mutations in dyschondrosteosis (Leri-Weill syndrome). Nat Genet. 1998 May. 19(1):67-9. [Medline].
- Boguszewski CL, Carlsson B, Carlsson LM. Mechanisms of growth failure in non-growth-hormone deficient children of short stature. Horm Res. 1997. 48 Suppl 4:19-22. [Medline].
- Cohen P, Bright GM, Rogol AD, et al. Effects of dose and gender on the growth and growth factor response to GH in GH-deficient children: implications for efficacy and safety. J Clin Endocrinol Metab. 2002 Jan. 87(1):90-8. [Medline]. [Full Text].
- de Mel T, Warnasooriya N, Fonseka C. Growth hormone deficiency in Sri Lanka: a preliminary study. Ceylon Med J. 1991 Sep. 36(3):95-7. [Medline].
- Elsas LJ, Endo F, Strumlauf E, Elders J, Priest JH. Leprechaunism: an inherited defect in a high-affinity insulin receptor. Am J Hum Genet. 1985 Jan. 37(1):73-88. [Medline].
- Ferry RJ Jr, Cohen P. The insulin-like growth factor axis in pediatrics. Clin Pediatr Endocrinol. 1999. 8(1):1-10.
- Gandrud LM, Wilson DM. Is growth hormone stimulation testing in children still appropriate?. Growth Horm IGF Res. 2004 Jun. 14(3):185-94. [Medline].
- Gatta V, Antonucci I, Morizio E, et al. Identification and characterization of different SHOX gene deletions in patients with Leri-Weill dyschondrosteosys by MLPA assay. J Hum Genet. 2007. 52(1):21-7. [Medline].
- GH Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab. 2000 Nov. 85(11):3990-3. [Medline]. [Full Text].
- Guyda HJ. Four decades of growth hormone therapy for short children: what have we achieved?. J Clin Endocrinol Metab. 1999 Dec. 84(12):4307-16. [Medline]. [Full Text].
- Guyda HJ. Growth hormone testing and the short child. Pediatr Res. 2000 Nov. 48(5):579-80. [Medline].
- Hardin DS, Woo J, Butsch R, Huett B. Current prescribing practices and opinions about growth hormone therapy: results of a nationwide survey of paediatric endocrinologists. Clin Endocrinol (Oxf). 2007 Jan. 66(1):85-94. [Medline].
- Harris NS, Crawford PB, Yangzom Y, et al. Nutritional and health status of Tibetan children living at high altitudes. N Engl J Med. 2001 Feb 1. 344(5):341-7. [Medline]. [Full Text].
- Horton WA, Hall JG, Scott CI, Pyeritz RE, Rimoin DL. Growth curves for height for diastrophic dysplasia, spondyloepiphyseal dysplasia congenita, and pseudoachondroplasia. Am J Dis Child. 1982 Apr. 136(4):316-9. [Medline].
- Horton WA, Rotter JI, Rimoin DL, Scott CI, Hall JG. Standard growth curves for achondroplasia. J Pediatr. 1978 Sep. 93(3):435-8. [Medline].
- Hunter AG, Hecht JT, Scott CI Jr. Standard weight for height curves in achondroplasia. Am J Med Genet. 1996 Mar 29. 62(3):255-61. [Medline].
- Lee MM. Clinical practice. Idiopathic short stature. N Engl J Med. 2006 Jun 15. 354(24):2576-82. [Medline].
- Li H, Leung SS, Lam PK, et al. Height and weight percentile curves of Beijing children and adolescents 0-18 years, 1995. Ann Hum Biol. 1999 Sep-Oct. 26(5):457-71. [Medline].
- Lyon AJ, Preece MA, Grant DB. Growth curve for girls with Turner syndrome. Arch Dis Child. 1985 Oct. 60(10):932-5. [Medline].
- March of Dimes. Fact sheets for achondroplasia. Available at: http://www.modimes.org. [Full Text].
- National Institute of Diabetes & Digestive Kidney Diseases. Fact sheets for patients on growth failure in chronic renal insufficiency. Available at: http://www.niddk.nih.gov. [Full Text].
- Palka G, Stuppia L, Guanciali Franchi P, et al. Short arm rearrangements of sex chromosomes with haploinsufficiency of the SHOX gene are associated with Leri-Weill dyschondrosteosis. Clin Genet. 2000 Jun. 57(6):449-53. [Medline].
- Palmer CG, Cronk C, Pueschel SM, et al. Head circumference of children with Down syndrome (0-36 months). Am J Med Genet. 1992 Jan 1. 42(1):61-7. [Medline].
- Parkin JM. Incidence of growth hormone deficiency. Arch Dis Child. 1974 Nov. 49(11):904-5. [Medline].
- Parks JS, Brown MR, Hurley DL, Phelps CJ, Wajnrajch MP. Heritable disorders of pituitary development. J Clin Endocrinol Metab. 1999 Dec. 84(12):4362-70. [Medline]. [Full Text].
- Prader A, Largo RH, Molinari L, Issler C. Physical growth of Swiss children from birth to 20 years of age. First Zurich longitudinal study of growth and development. Helv Paediatr Acta Suppl. 1989 Jun. 52:1-125. [Medline].
- Quigley CA, Gill AM, Crowe BJ, et al. Safety of growth hormone treatment in pediatric patients with idiopathic short stature. J Clin Endocrinol Metab. 2005 Sep. 90(9):5188-96. [Medline].
- Rao E, Weiss B, Fukami M, et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nat Genet. 1997 May. 16(1):54-63. [Medline].
- Roche AF, Wainer H, Thissen D. The RWT method for the prediction of adult stature. Pediatrics. 1975 Dec. 56(6):1027-33. [Medline].
- Rosenbloom AL, Almonte AS, Brown MR, et al. Clinical and biochemical phenotype of familial anterior hypopituitarism from mutation of the PROP1 gene. J Clin Endocrinol Metab. 1999 Jan. 84(1):50-7. [Medline]. [Full Text].
- Rosenfeld RG. Transition from pediatric to adult care for growth hormone deficiency. J Pediatr Endocrinol Metab. 2003 May. 16 Suppl 3:645-9. [Medline].
- Saenger P. Partial growth hormone insensitivity--idiopathic short stature is not always idiopathic. Acta Paediatr Suppl. 1999 Feb. 88(428):194-8. [Medline].
- Satin-Smith MS, Katz LL, Thornton P, Gruccio D, Moshang T Jr. Arm span as measurement of response to growth hormone (GH) treatment in a group of children with meningomyelocele and GH deficiency. J Clin Endocrinol Metab. 1996 Apr. 81(4):1654-6. [Medline].
- Savendahl L, Davenport ML. Delayed diagnoses of Turner's syndrome: proposed guidelines for change. J Pediatr. 2000 Oct. 137(4):455-9. [Medline].
- Shanske AL, Puri M, Marshall B, Saenger P. Unique deletion in exon 5 of SHOX gene in a patient with idiopathic short stature. Horm Res. 2007. 67(2):61-6. [Medline].
- Shears DJ, Vassal HJ, Goodman FR, et al. Mutation and deletion of the pseudoautosomal gene SHOX cause Leri-Weill dyschondrosteosis. Nat Genet. 1998 May. 19(1):70-3. [Medline].
- Tanner JM, Goldstein H, Whitehouse RH. Standards for children's height at ages 2-9 years allowing for heights of parents. Arch Dis Child. 1970 Dec. 45(244):755-62. [Medline].
- Toledo C, Alembik Y, Aguirre Jaime A, Stoll C. Growth curves of children with Down syndrome. Ann Genet. 1999. 42(2):81-90. [Medline].
- Turner's Syndrome Society. Ulrich-Turner syndrome. Available at: http://www.turner-syndrome-us.org/. [Full Text].
- Weinzimer SA, Homan SA, Ferry RJ, Moshang T. Serum IGF-I and IGFBP-3 concentrations do not accurately predict growth hormone deficiency in children with brain tumours. Clin Endocrinol (Oxf). 1999 Sep. 51(3):339-45. [Medline].
- Westphal O, Lindberg A. Final height in Swedish children with idiopathic growth hormone deficiency enrolled in KIGS treated optimally with growth hormone. Acta Paediatr. 2008 Dec. 97(12):1698-706. [Medline].
- Wilson DM, Frane J. A brief review of the use and utility of growth hormone stimulation testing in the NCGS: do we need to do provocative GH testing?. Growth Horm IGF Res. 2005 Jul. 15 Suppl A:S21-5. [Medline].
- [Guideline] Wilson TA, Rose SR, Cohen P, Rogol AD, Backeljauw P, Brown R. Update of guidelines for the use of growth hormone in children: the Lawson Wilkins Pediatric Endocrinology Society Drug and Therapeutics Committee. J Pediatr. 2003 Oct. 143(4):415-21. [Medline].
- Sandberg DE, Gardner M. Short Stature: Is It a Psychosocial Problem and Does Changing Height Matter?. Pediatr Clin North Am. 2015 Aug. 62 (4):963-82. [Medline].
- Zayed S, Madlon-Kay DJ. Growth Hormone for Treatment of Idiopathic Short Stature in Children. Am Fam Physician. 2015 Jul 1. 92 (1):64. [Medline].
- Wit JM, Oostdijk W. Novel approaches to short stature therapy. Best Pract Res Clin Endocrinol Metab. 2015 Jun. 29 (3):353-66. [Medline].
Proper use of a wall-mounted stadiometer.
Comparison of the growth patterns between idiopathic short stature and constitutional growth delay.
Bone age comparison between an 8-year-old boy (left) and a 14-year-old adolescent boy (right).
Growth chart for Turner syndrome. Note that the upper limit overlaps the range for girls of normal height.
A single, central, maxillary incisor reflects a defect in midline facial development.

















